- Product Details
Keywords
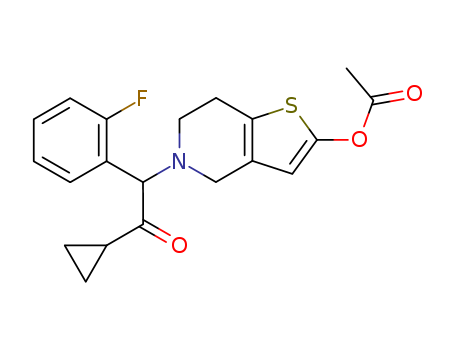
- Prasugrel
- Prasugrel powder
- 150322-43-3
Quick Details
- ProName: Pharmaceutical Grade Prasugrel Raw Mat...
- CasNo: 150322-43-3
- Molecular Formula: C20H20FNO3S
- Appearance: White powder
- Application: Prasugrel is used in combination with ...
- DeliveryTime: Abiut 10 days
- PackAge: Aluminum foil bag,fluorinated bottles,...
- Port: Shenzhen port
- Purity: 99%
- Storage: Store in cool & dry place. Keep away f...
- Transportation: 1) Door to Door Service by DHL/FEDEX/E...
- LimitNum: 0
- Moisture Content: <0.1%
- Impurity: <0.1%
Superiority
1) Provide costomers with "one-stop"packaging service,from research,development,production,export and so on.
2) We have our own R&D and production base,equipped with advanced production equipment and precision testing the Unitef States Pharmacopoeia(USP),the Britis Pharmacopoeia(EP) and other international advanced standards.All products are through the KOSGER,HALAL certification,the ISO quality management system certification and the HACCP certification.
3) More than 12 years of export experience.
4)Competitive price in China market.
Details
Prasugrel was approved for use in Europe in February 2009, and in the US in July 2009, for the reduction of thrombotic cardiovascular events (including stent thrombosis) in people with acute coronary syndrome (ACS) who are to be managed with percutaneous coronary intervention (PCI).